A few words about β-peptides¶
Structurally speaking, the difference between β-amino acids and their natural counterparts is only a methylene group in the backbone. While they have many structural functional and properties in common with α-peptides and proteins, the additional torsion angle lends these molecules unique and interesting features, simultaneously rendering common molecular modeling tools developed for proteins unusable.
The present force field extension deals with acyclic β-amino acids and the peptides constructed from them. According to the substitution site, four common variants exist:
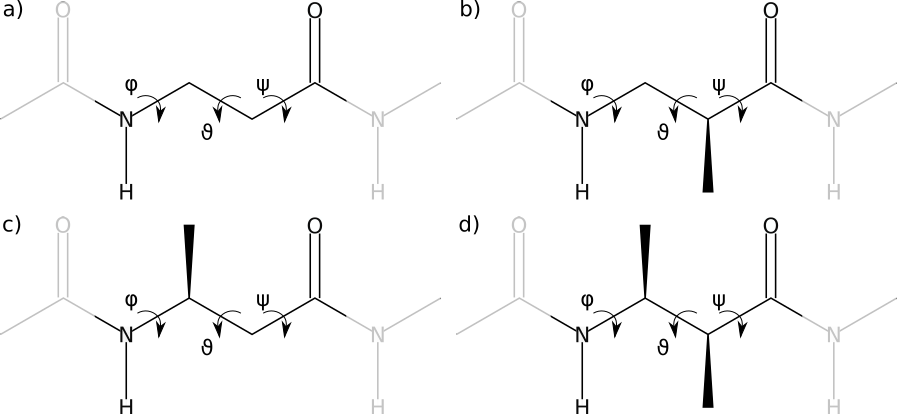
The four kinds of β-amino acids: bare β-backbone or β-alanine (a), β2-amino acid (b), β3-amino acid (c) and β2,3-amino acid (d)
In contrast to natural ones, β-amino acids do not have a single, universally accepted terminology. In the documentation of this force field extension, we adopt the following, widely used convention, which emphasizes the homology with α-amino acids and allows to account for the absolute conformation (chirality) as well. The general block format is :
<chirality><substitution type>h<side-chain designation>, where:
- <chirality>
- is the designation of the chirality of the substitution site atoms. For monosubstituted β-amino acids, this is either (S) or (R), including parentheses. For disubstituted ones, the substitution site is also labelled to avoid ambiguity, i.e. (2S, 3R) etc.
- <substitution type>:
- either β2, β3or β2,3.
- <side-chain designation>:
- single-letter abbreviation of the proteinogenic amino-acid whose side-chain is referred to. As for the chirality above, in the case of disubstituted amino-acids the substitution site must be explicitly given in order to avoid confusion, i.e. (2A,3Q), etc.
We include α-amino acids in this notation, too, with the following scheme:
<chirality>α<side-chain designation>, where:
- <chirality>
- is similar as for β2- or β3-amino acids, i.e. either (S) or (R)
- <side-chain designation>:
- is once again the single-letter abbreviation of proteinogenic amino acids
- Examples:
- monosubstituted:
- (S)β2hV: valine side-chain on the α-carbon with S chirality
- (R)β3hR: arginine side-chain on the β-carbon with R chirality
- disubstituted:
- (2S,3R)β2,3 h(2A,3L): disubstituted β-amino acid with an alanine side-chain on the α-carbon (with S chirality) and an arginine on the β-carbon (R chirality)
- achiral (bare backbone):
- βA
- α-amino acids:
- (S)αV: L-valine
- (R)αW: D-tryptophan
Two, more complicated examples are shown in the next two figures:
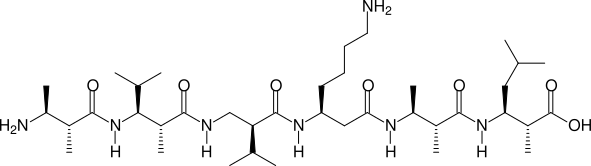
(2R,3S)β2,3h(2A,3A) - (2R,3S)β2,3h(2A,3V) - (S)β2hV - (S)β3hK - (2R,3S)β2,3h(2A,3A) - (2R,3S)β2,3h(2A,3L)
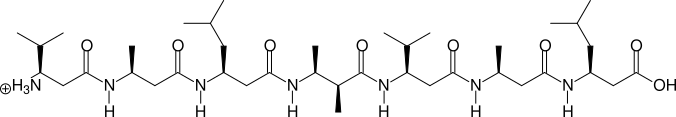
(S)β3hV - (S)β3hA - (S)β3hL - (2S,3S)β2,3h(2A,3A) - (S)β3hV - (S)β3hA - (S)β3hL